43 bohr diagram for fluorine
PDF How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He [Solved] Imagine a Bohr-Rutherford diagram of a fluorine ... Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. Set up the diagram. To set up the diagram, you will need a circle in the middle. Add in orbitals and electrons.
Bohr Diagram Worksheet Word Doc.docx - NAME Period BOHR ... There are 4 shells in the Bohr model of a hydrogen atom. 6) How many valence electrons are there in a fluorine atom? There are 7 valence electrons in a fluorine atom. 7) How many shells are there in the Bohr model of a silicon atom? There are 3 shells in the Bohr model of a silicon atom. 8) Fill in the blanks beside each Bohr model diagram.
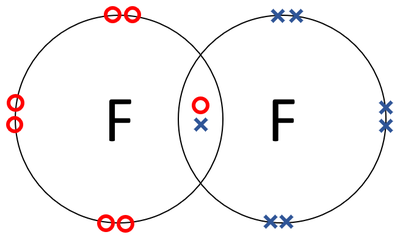
Bohr diagram for fluorine
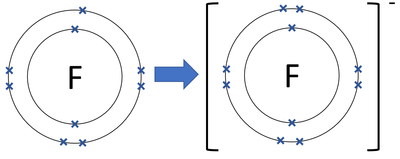
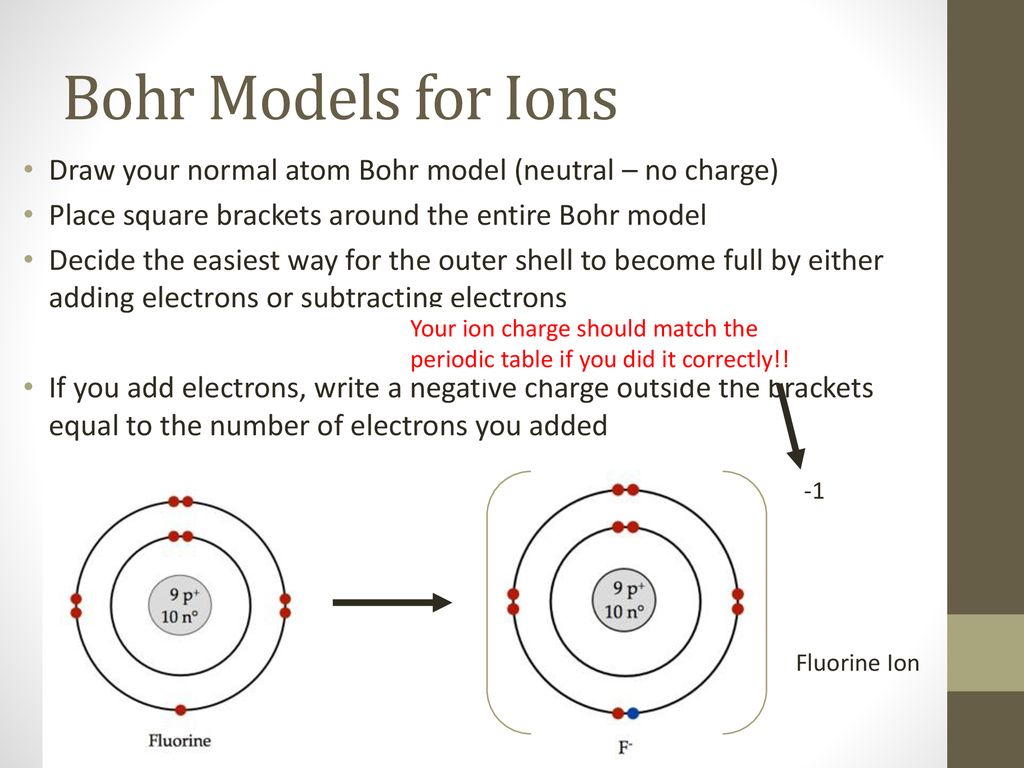
› chemistry › shapes-of-orbitalsShapes of Orbitals | What is Orbital? Types of Orbitals From the above diagram, it is clear that for 2s orbital, there will be 2 maxima in the R 2/ 2 Vs r plot. It will be one at r= 0 and the other one at nearly 2= 210 pm, between these two maxima the probability becomes zero at about r= 105 pm. This is called the nodal point. The size of the 2s orbital is larger than that of the 1s orbital. Fluorine Bohr Model - How to draw Bohr diagram for ... According to the Bohr diagram of Fluorine, the outer shell is L-shell which contains 7 valence electrons. Properties of Fluorine At standard conditions, it appears as a pale yellow diatomic gas. It is an extremely reactive and the most electronegative element in chemistry. It has a cubic crystal structure. PDF Warm Up 1. Draw a Bohr diagram of a fluorine atom. 2. Draw ... Draw a Bohr diagram of a fluorine atom. 2. Draw a Bohr diagram of a magnesium ion. Compounds using diagrams 1.notebook 2 March 20, 2019 _____: an atom that now has a positive or negative charge because it gained or lost a specific number of electrons. ...
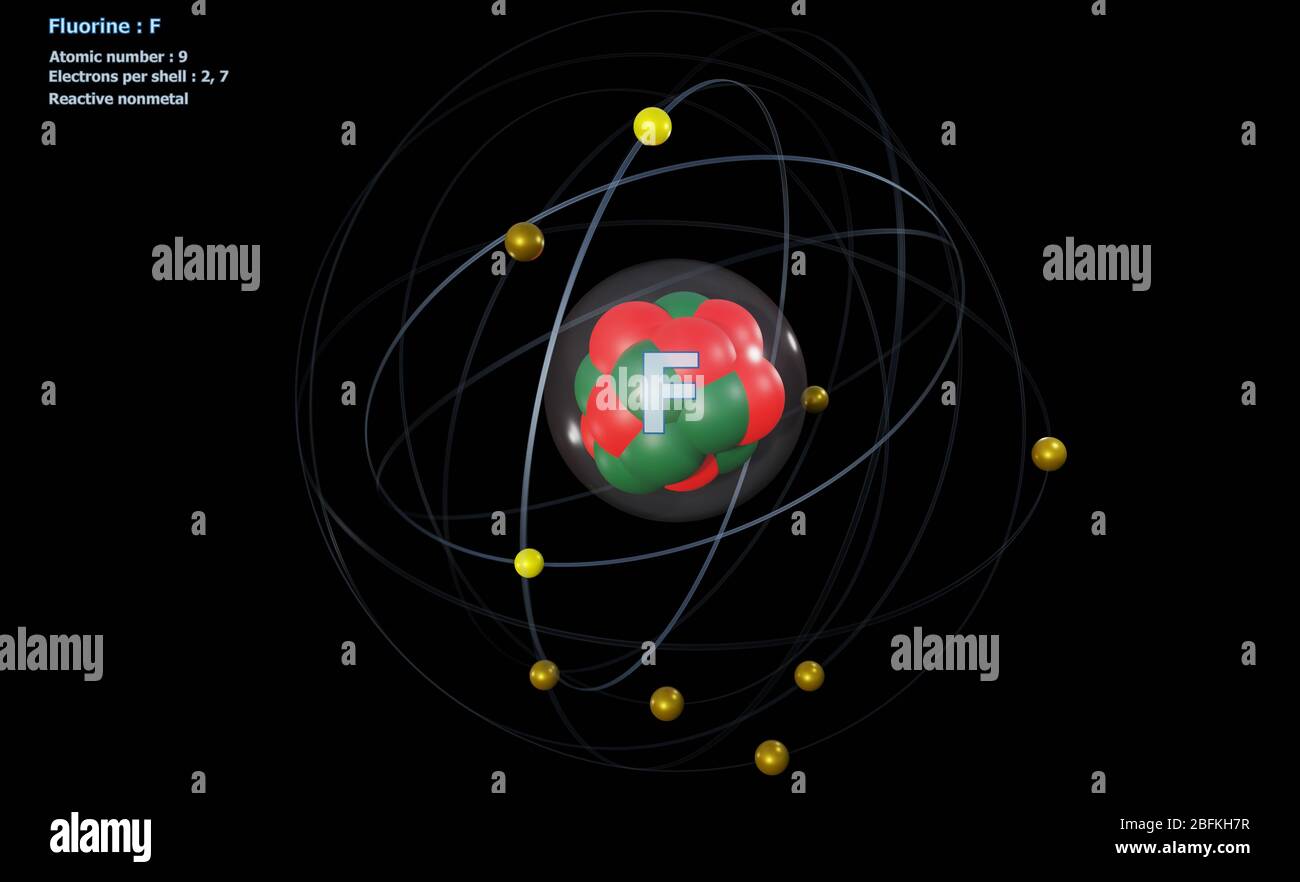
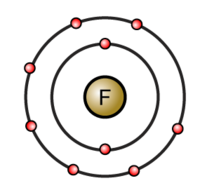
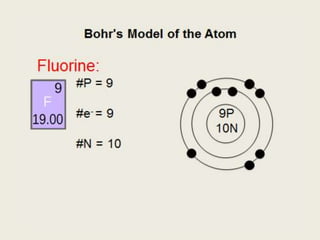
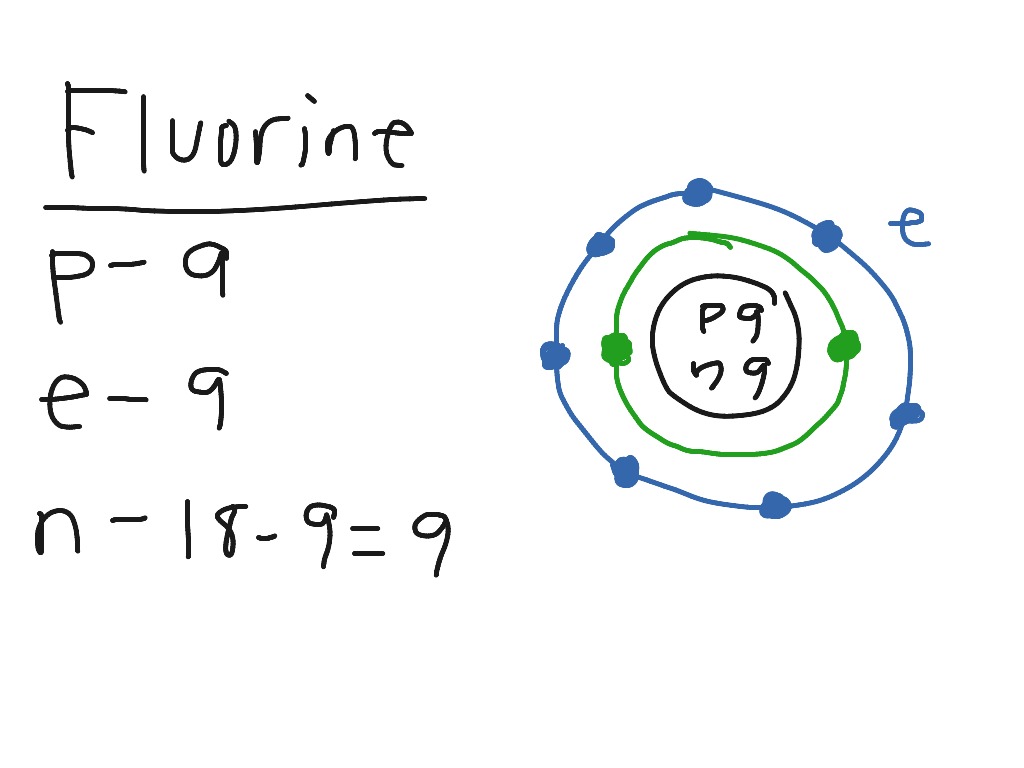
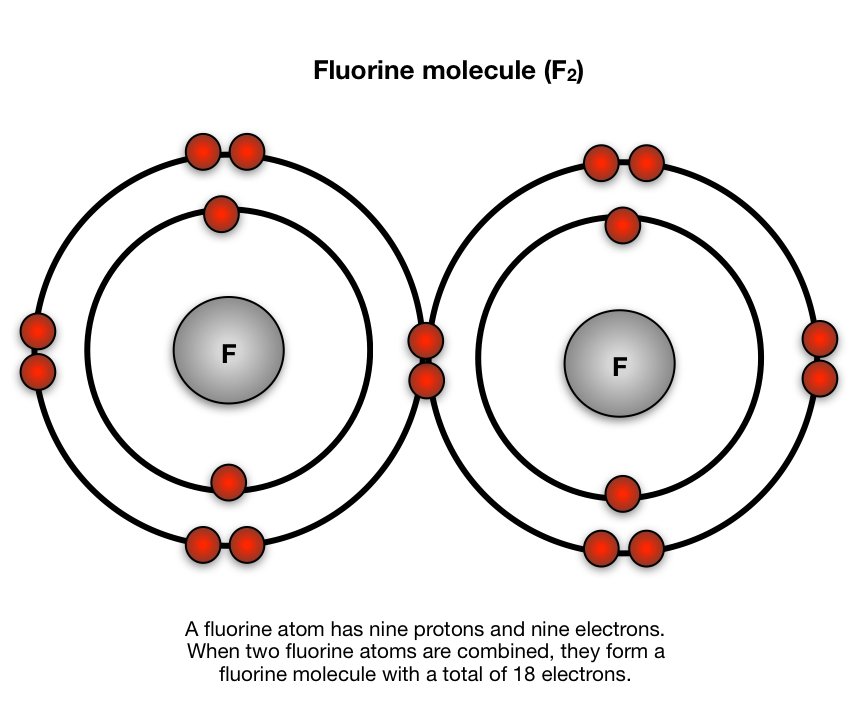
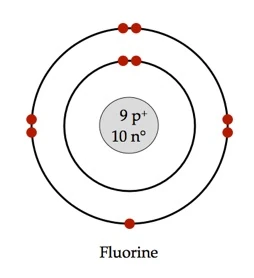
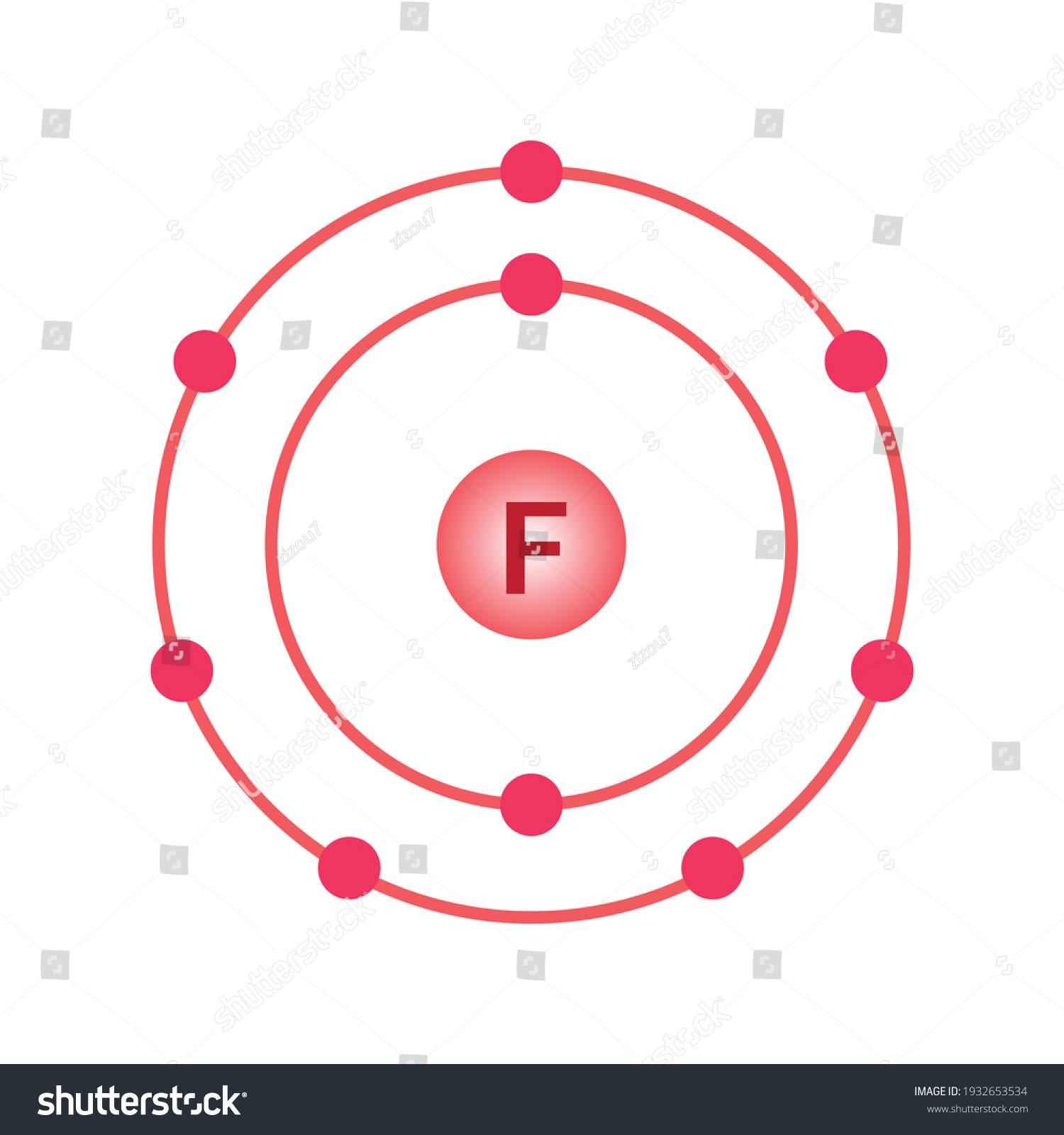
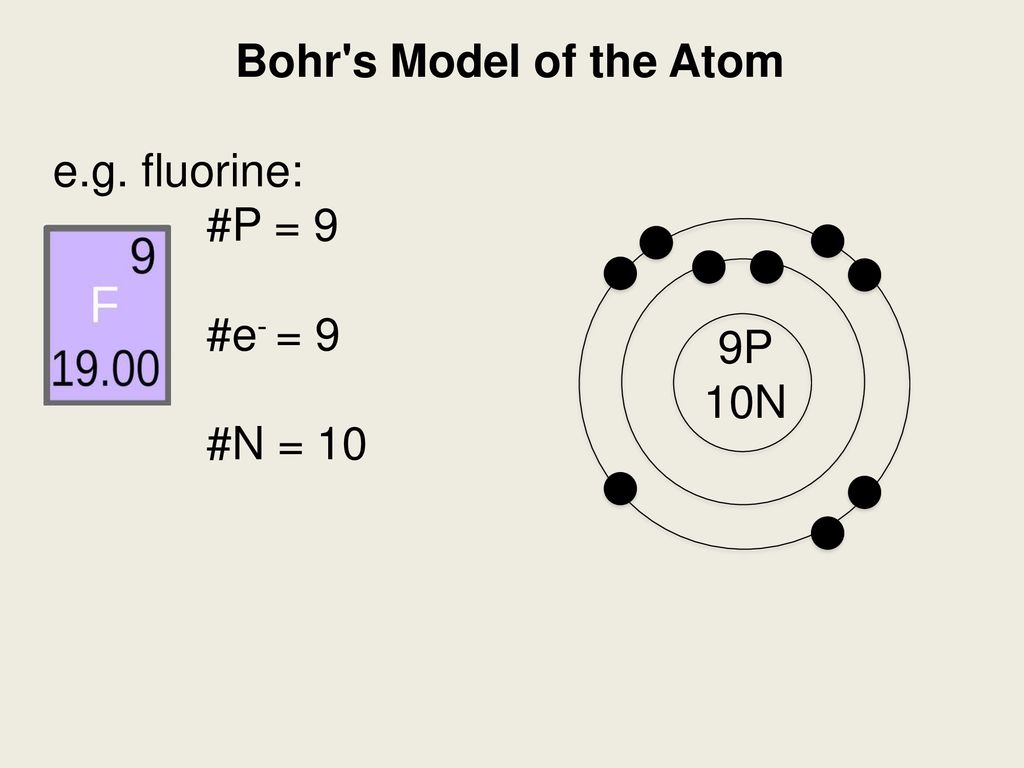
Bohr diagram for fluorine. ATOMIC STRUCTURE - users.stlcc.edu According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons. Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell. (a) Draw the Bohr-Rutherford diagram (without neutrons ... Find step-by-step Biology solutions and your answer to the following textbook question: (a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion. blog.prepscholar.com › atomic-radius-trendUnderstanding Atomic Radius Trends: The 2 Key Principles Feb 07, 2021 · Comparing carbon (C) with an atomic number of 6 and fluorine (F) with an atomic number of 9, we can tell that, based on atomic radius trends, a carbon atom will have a larger radius than a fluorine atom since the three additional protons the fluorine has will pull its electrons closer to the nucleus and shrink the fluorine's radius. And this is ...
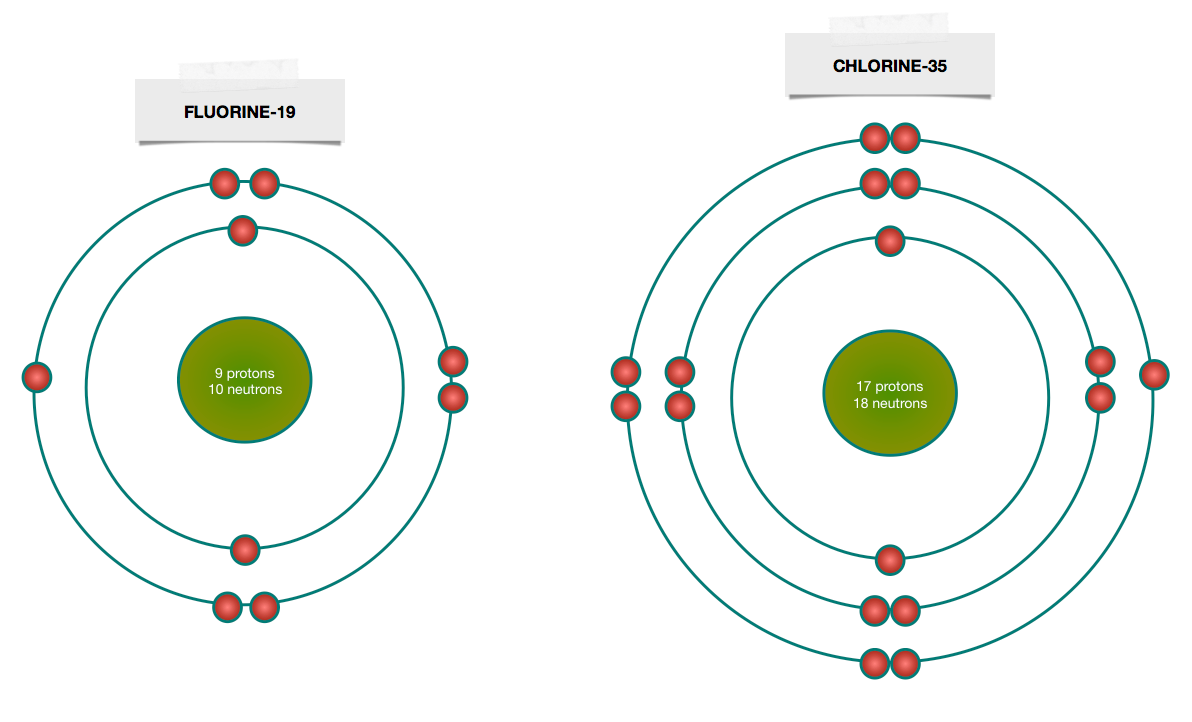
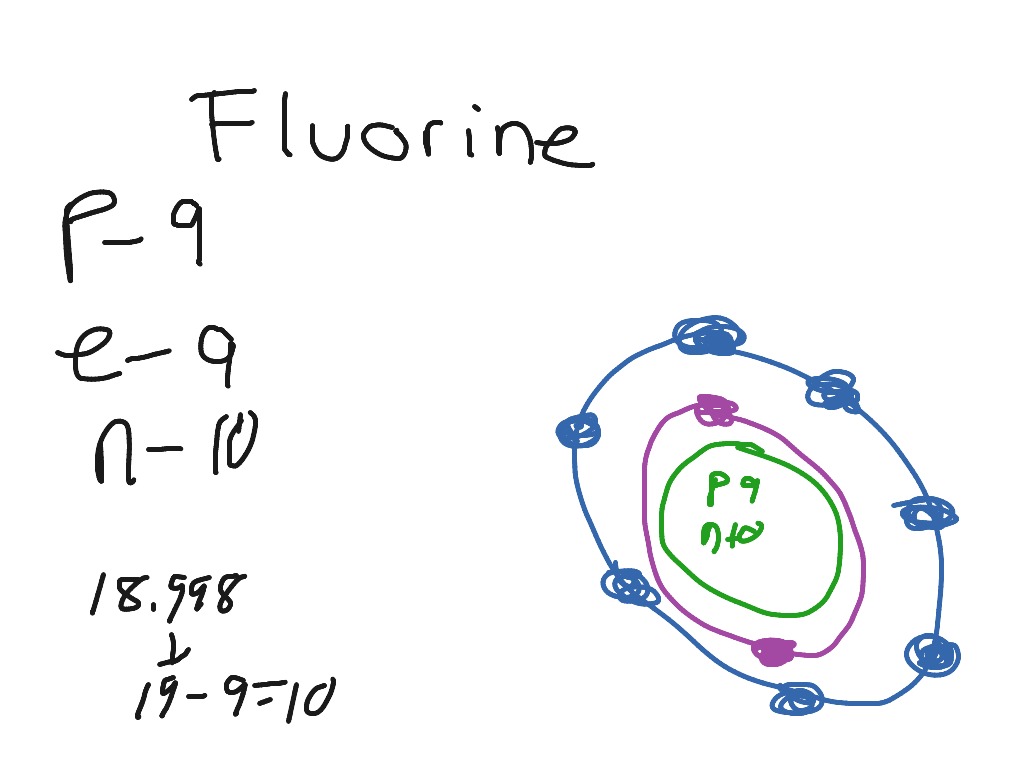
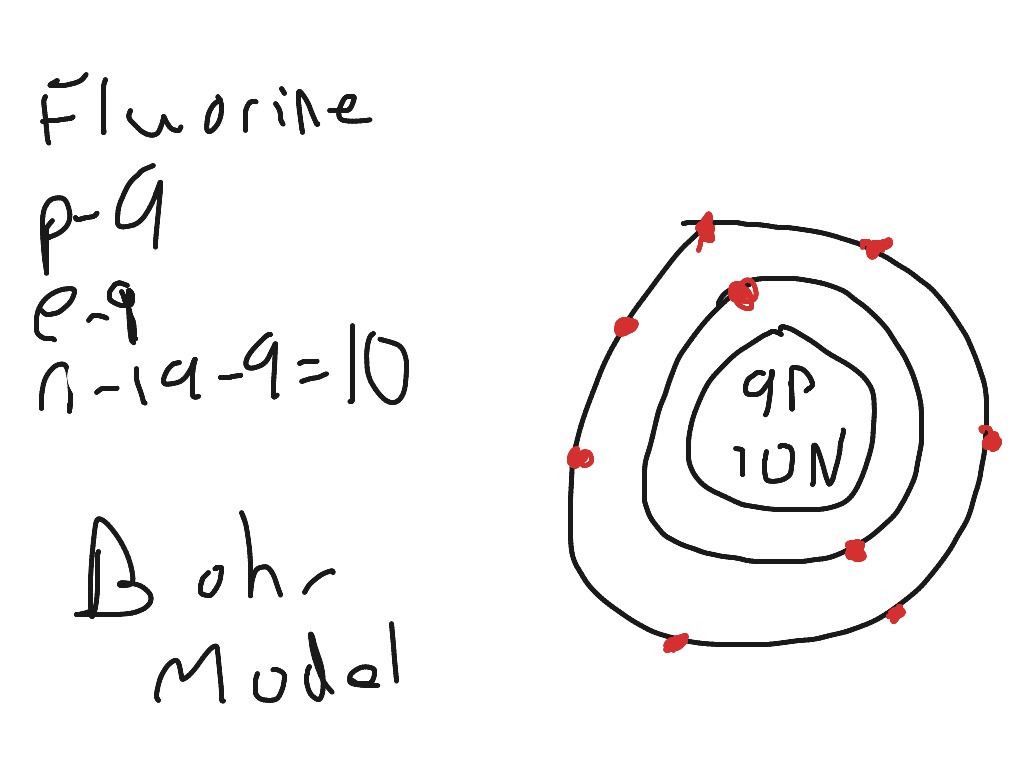
Bohr diagram for fluorine? - Answers Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass number of 19). There will be an equal number... Bohr Diagram Of Flourine - schematron.org Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass.Figure \ (\PageIndex {2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell. Bohr Rutherford Diagram For The First 20 Elements In this model of the atom, the electrons travel around the nucleus in well-defined Steps for Drawing Bohr Diagrams (for use only with the first 20 elements). 1. Bohr Models First 20 Elements in the Periodic Table. First 20 .. Using the Main Group Elements of the Periodic Table to Draw Bohr-Rutherford Diagrams He. Solved 10. Imagine a Bohr-Rutherford diagram of a fluorine ... 1. What do the diagrams have in common? 2. When might it be most helpful to use a Bohr- Rutherford diagram? 3. When might you choose to use a Lewis symbol instead of a Bohr-Rutherford diagram? Question: 10. Imagine a Bohr-Rutherford diagram of a fluorine atom and a Lewis symbol of the same atom. 1. What do the diagrams have in common? 2.
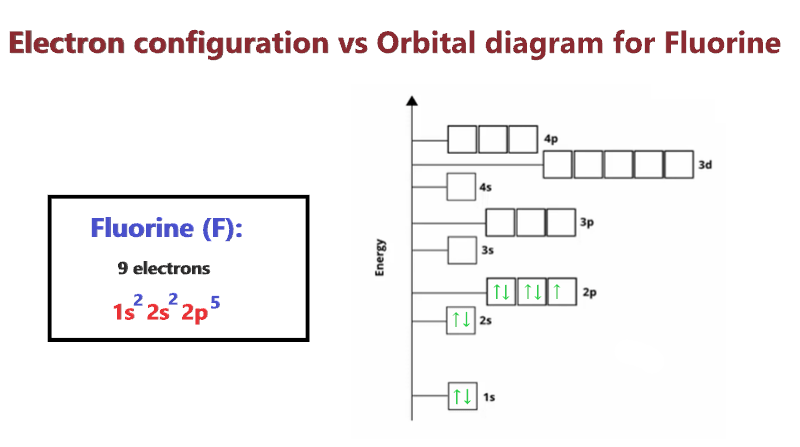
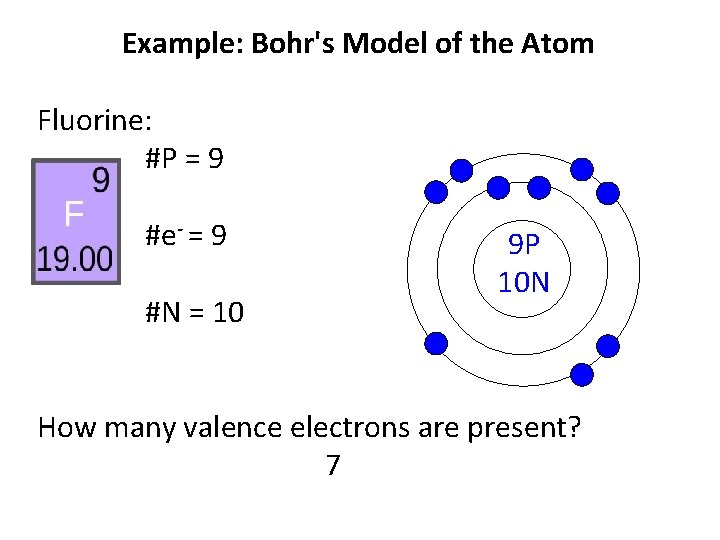
PPTX Bohr Models Bohr Model diagrams are used to represent the electron structures of elements. Protons and neutrons are placed in the middle to represent the nucleus. Electrons are placed in the electron shells. Making a Bohr Model. Using the example Fluorine, we will work through the steps in creating a Bohr model. › cbse › important-questions-class-9Important Questions for CBSE Class 9 Science Chapter 4 ... The diagram of experiment is as follows: (Image will be uploaded soon) 36. Write the postulates of Bohr theory? Ans: The postulate of Bohr’s theory is as follows: An electron revolves around the nucleus in the orbit of an atom in a definite path known as orbits or shells. Energy of each orbit is fixed. Fluorine(F) electron configuration and orbital diagram Orbital Diagram Fluorine Electron configuration of fluorine in the excited state Atoms can jump from one orbital to another orbital in the excited state. This is called quantum jump. The ground-state electron configuration of fluorine is 1s 2 2s 2 2p 5. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. study.com › learn › bohr-model-questions-and-answersBohr Model Questions and Answers | Study.com Bohr Model Questions and Answers. Get help with your Bohr model homework. Access the answers to hundreds of Bohr model questions that are explained in a way that's easy for you to understand.
Bohr Diagram For Fluorine - schematron.org According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 .
How to Draw the Bohr-Rutherford Diagram of Fluorine - YouTube Fluorine has 2 electrons in its first shell and 7 in its second.Check me out:
Solved Draw a Bohr model for elements with atomic ... - Chegg Draw a Bohr model for elements with atomic numbers 1-18 (Hydrogen thru Argon). Draw each diagram neatly, clearly labeling the correct number of protons, electrons, and neutrons for each diagram. Use the average atomic mass from the periodic table for the mass number (round to the nearest whole number).
What is the Bohr model of fluorine? - FindAnyAnswer.com A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Who created the Bohr model? Niels Bohr
PDF Bohr diagram for sodium chloride In the Bohr model, electrons are displayed as traveling in circles in different shells, depending on which element you have. Figure \(\PageIndex{2}\) contrast bohr diagrams for lithium, fluorine, and aluminum atoms. The shell closest to the core is called the K shell, the next is the L shell, the next is the M shell.
Atomic Structure (Bohr Model) for Fluorine (F - YouTube In this video we'll look at the atomic structure and Bohr model for the Fluorine atom (F). We'll use a Bohr diagram to visually represent where the electrons...
Bohr Model of all Elements (Diagrams + Chart Inside) Bohr Model of all Elements (Diagrams + Chart) Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Bohr model of the atom - Chemistry Resource Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons. In the second orbit, there are 7 electrons.
PDF 27 Bohr Diagrams - Mr. Smith's Website Fluorine should have 9 electrons. The first orbit will hold 2 (the maximum it can hold) and the remaining 7 will go in the second orbit. Example 2 Draw a Bohr diagram of a single atom of magnesium. 12 24Mg Homework Draw Bohr diagrams for the first 18 elements on the periodic table (Atomic numbers 1 to 18).
techiescientist.com › sf6-lewis-structureSF6 Lewis Structure, Molecular Geometry ... - Techiescientist 2 days ago · Molecular Geometry and MO Diagram of SF6 There are Fluorine atoms all around Sulfur which gives the compound a kind of symmetry when we look at it on a planar level. When we look at the molecular geometry of any compound we get to see a 3-D image of how the atoms are distributed which we cannot identify while making a Lewis Structure.
› cms › libAtomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
PDF Warm Up 1. Draw a Bohr diagram of a fluorine atom. 2. Draw ... Draw a Bohr diagram of a fluorine atom. 2. Draw a Bohr diagram of a magnesium ion. Compounds using diagrams 1.notebook 2 March 20, 2019 _____: an atom that now has a positive or negative charge because it gained or lost a specific number of electrons. ...
Fluorine Bohr Model - How to draw Bohr diagram for ... According to the Bohr diagram of Fluorine, the outer shell is L-shell which contains 7 valence electrons. Properties of Fluorine At standard conditions, it appears as a pale yellow diatomic gas. It is an extremely reactive and the most electronegative element in chemistry. It has a cubic crystal structure.
› chemistry › shapes-of-orbitalsShapes of Orbitals | What is Orbital? Types of Orbitals From the above diagram, it is clear that for 2s orbital, there will be 2 maxima in the R 2/ 2 Vs r plot. It will be one at r= 0 and the other one at nearly 2= 210 pm, between these two maxima the probability becomes zero at about r= 105 pm. This is called the nodal point. The size of the 2s orbital is larger than that of the 1s orbital.


















Comments
Post a Comment