40 helium orbital diagram
Helium Energy Levels - Georgia State University Helium Energy Levels The helium ground state consists of two identical 1s electrons. The energy required to remove one of them is the highest ionization energy of any atom in the periodic table: 24.6 electron volts. The energy required to remove the second electron is 54.4 eV, as would be expected by modeling it after the hydrogen energy levels.The He+ ion is just like a hydrogen atom with two ... 9.8: Molecular Orbital Theory - Chemistry LibreTexts 20.02.2022 · Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those …
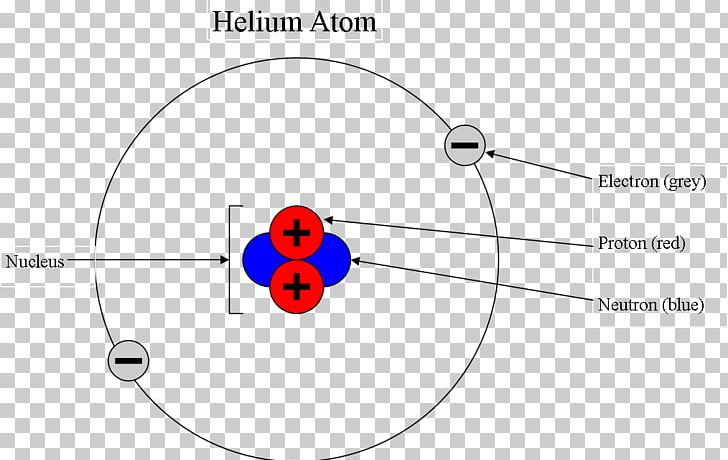
Bohr model - Wikipedia In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.It came after the solar system Joseph Larmor model (1897), the …

Helium orbital diagram
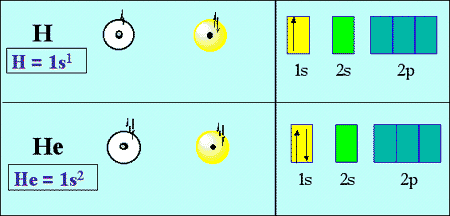
Draw the electron configuration diagram for helium? Question Draw the electron configuration diagram for helium? Medium Solution Verified by Toppr Helium has 2 valence electrons, located in period 1, group 18 and in S-block. Therefore its electron configuration is, 1s 2. Was this answer helpful? 0 0 Get the Free Answr app Click a picture with our app and get instant verified solutions Scan Me OR Helium hydride ion - Wikipedia The helium hydride ion or hydridohelium(1+) ion or helonium is a cation (positively charged ion) with chemical formula HeH +.It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang. Electron configuration for Helium (element 2). Orbital diagram Below is the electronic diagram of the Helium atom Distribution of electrons over energy levels in the He atom 1-st level (K): 2 Valence electrons of Helium The number of valence electrons in a Helium atom - 2. Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
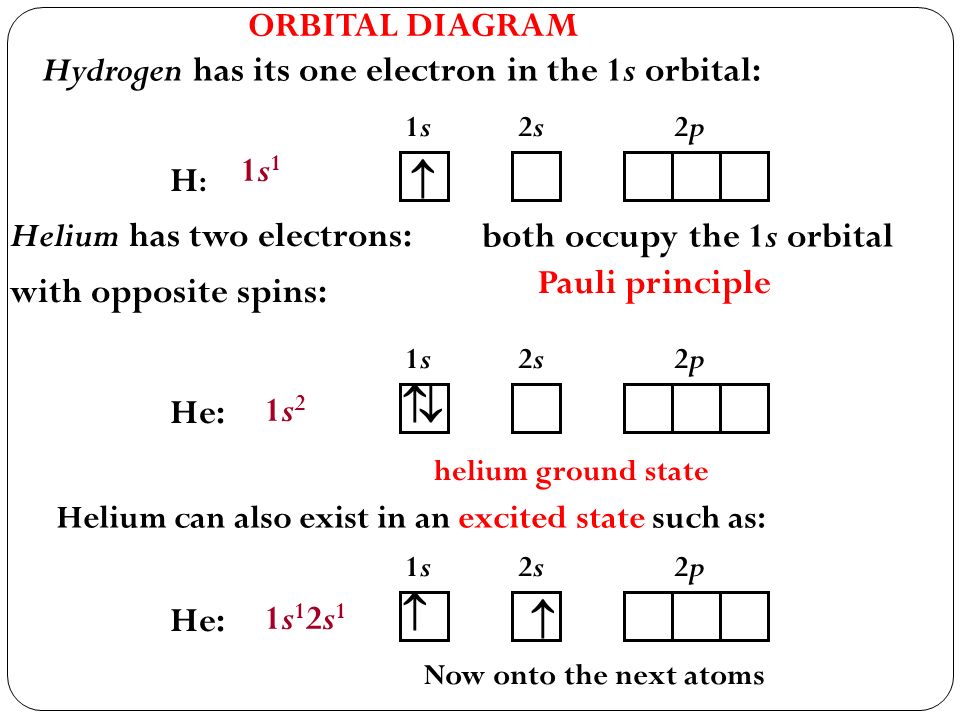
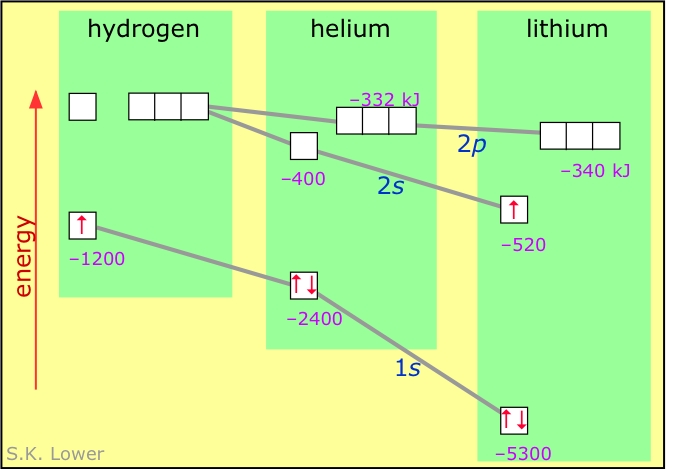
Helium orbital diagram. Hund's Rule and Orbital Filling Diagrams | Chemistry for ... The orbital filling diagrams for hydrogen, helium, and lithium are shown in Figure below. Figure 2. Orbital filling diagrams for hydrogen, helium, and lithium. According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. Molecular Orbital Diagram of Helium Molecule - Nature of ... Molecular Orbital Diagram of Helium Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.W... Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
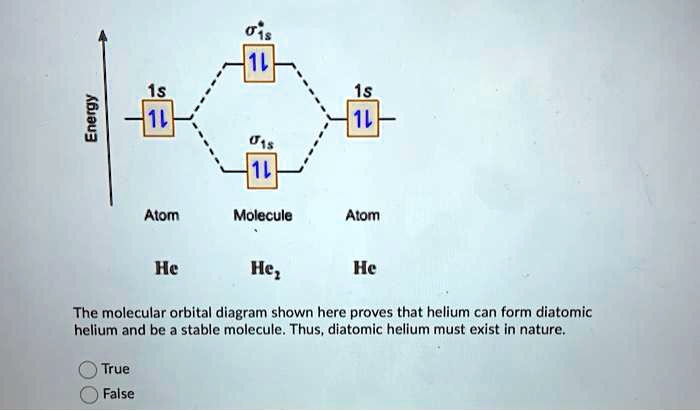
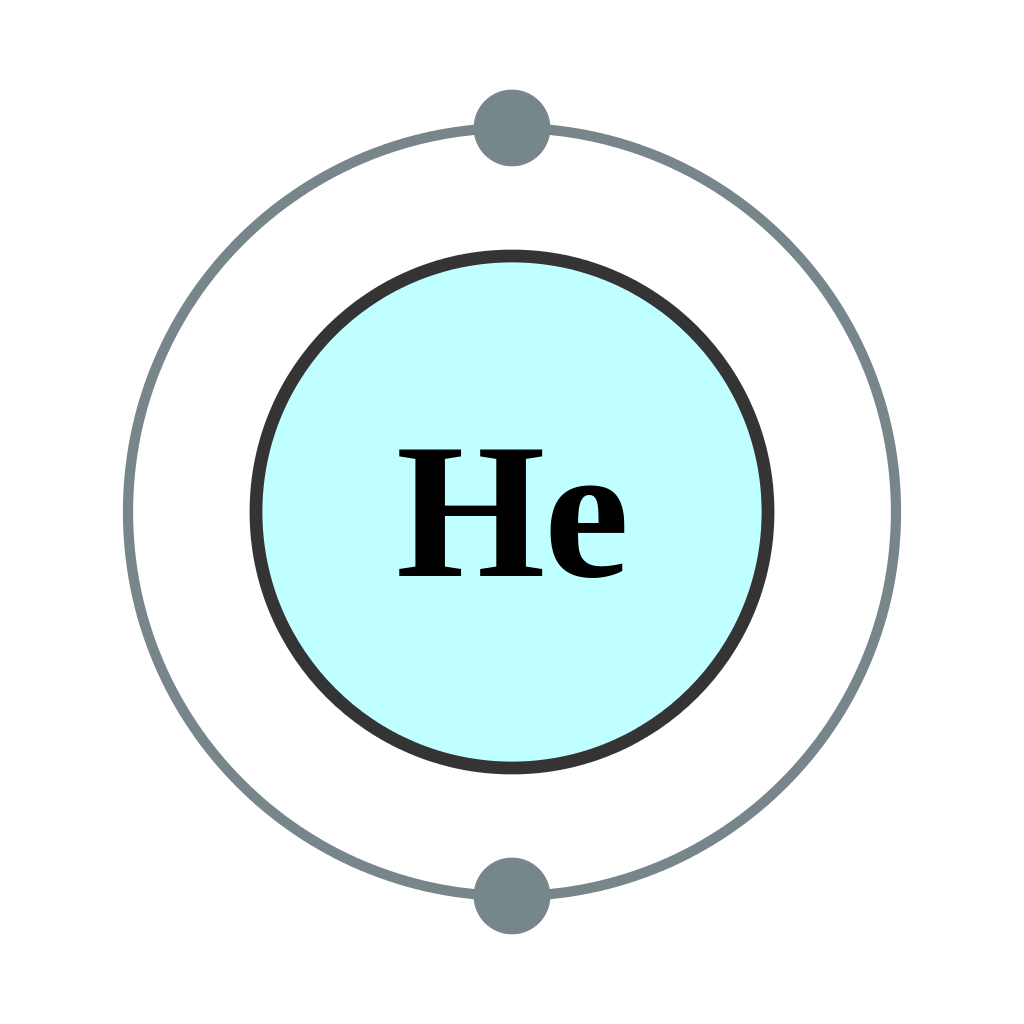
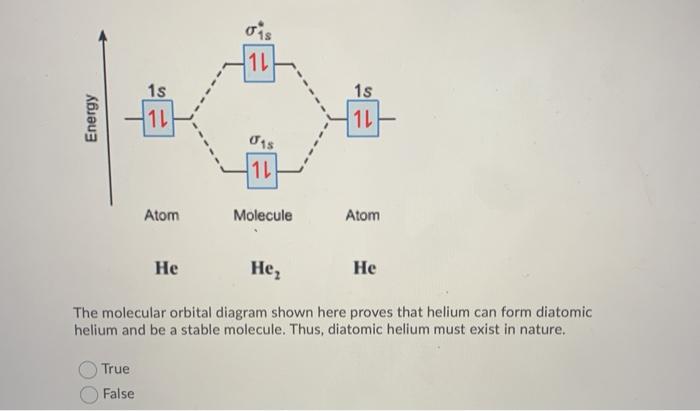
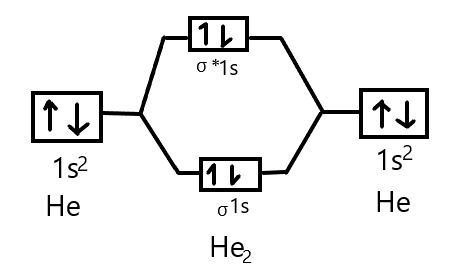
Chem4Kids.com: Helium: Orbital and Bonding Info So... for the element of HELIUM, you already know that the atomic number tells you the number of electrons. That means there are 2 electrons in a helium atom. Looking at the picture, you can see there are two electrons in shell one. That means the first shell is full. More about the history and places to find helium. Electron Configuration for Helium (He) Live. •. Helium only has 2 electrons and therefore it has a configuration of 1s 2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas. This means it will not react with other atoms. Helium orbital diagram - Big Chemical Encyclopedia Helium orbital diagram Arrows are added to an orbital diagram to show the distribution of electrons in the possible orbitals and the relative spin of each electron. The following is an orbital diagram for a helium atom. [Pg.424] A helium atom, for example, has two electrons. The electron configuration and orbital diagram for helium are ... [Pg.298] 9.8: Molecular-Orbital Theory Does not Predict a Stable ... The bonding in any diatomic molecule with two He atoms can be described using the following molecular orbital diagram: B The He 22+ ion has only two valence electrons (two from each He atom minus two for the +2 charge). We can also view He 22+ as being formed from two He + ions, each of which has a single valence electron in the 1 s atomic orbital.
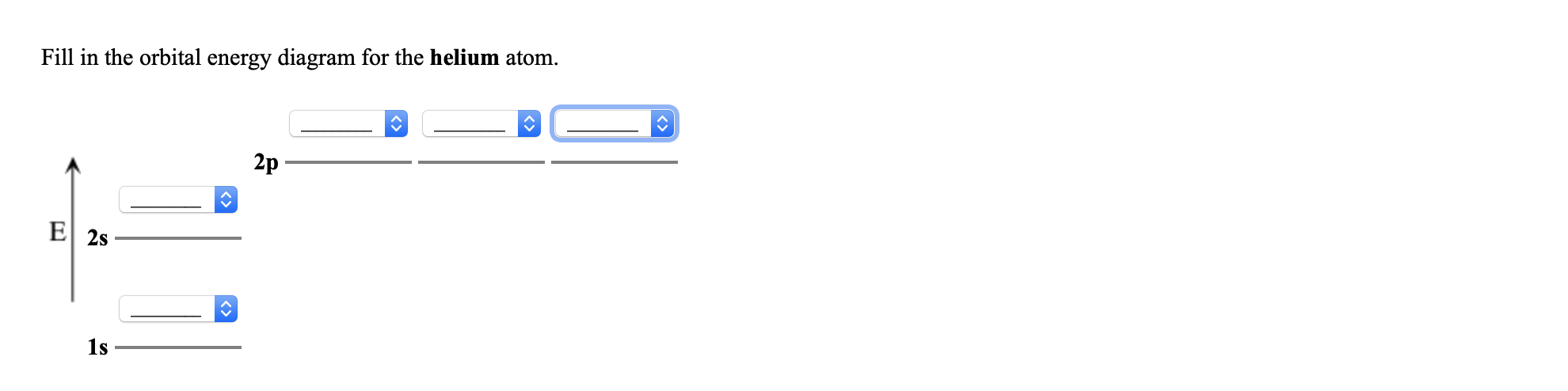
Consider the following partial orbital energy diagram ... Chemistry questions and answers. Consider the following partial orbital energy diagram for helium shown below. E (kJ/mol) E3a = -146.0 3d Ess=-160.8 3s E2p=-325.1 2p E2s = ?? 2s a) When an electron transitions from the 3d to the 2s orbital, 501.8 nm light is emitted. Based on this information and the diagram above, what is the energy, in kJ/mol ... What is the orbital diagram for Helium? - AskingLot.com What is the orbital diagram for Helium? Helium only has 2 electrons and therefore it has a configuration of 1s2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas. Shells, subshells, and orbitals (video) - Khan Academy The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have different energies. Molecular Orbital Theory - Purdue University Using the Molecular Orbital Model to Explain Why Some Molecules Do Not Exist. This molecular orbital model can be used to explain why He 2 molecules don't exist. Combining a pair of helium atoms with 1s 2 electron configurations would produce a molecule with a pair of electrons in both the bonding and the * antibonding molecular orbitals. The total energy of an …
PDF Exercise 2.3 Molecular Orbital Energy Level Diagrams ... Molecular Orbital Energy Level Diagrams: Hydrogen and Helium "Count and Sort" Algorithm for Building Molecular Orbital Energy Level Diagrams 1. Write the electron configuration for each atom , and count the total number of valence electrons. If you're working with an ion, adjust the valence electron count according to the charge. 2.
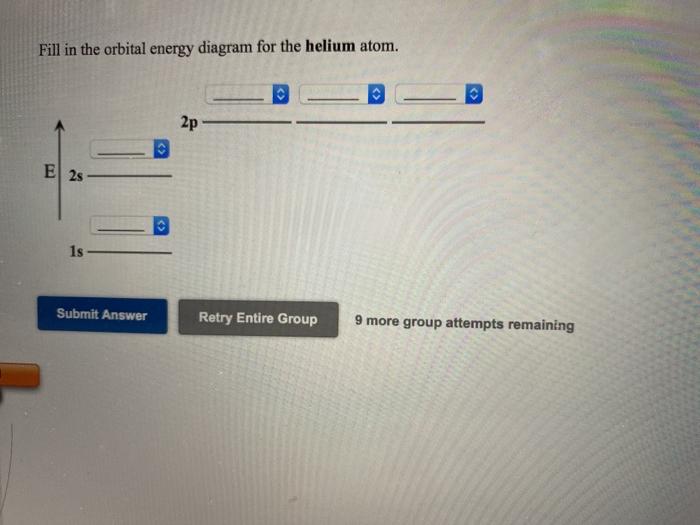
Arrangements of electrons in the orbitals of an atom is ... The orbital diagram for helium is, So while hydrogen has the electron configuration of 1s 1 , helium has the electron configuration of 1s 2. The energy diagram for helium is shown as here. Notice that there has been a change in the relative energies of the 2s and 2p orbitals. This is an important point that must be addressed at this point.
Molecular Orbital Diagram For He2 - schematron.org Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total Since the bond order is zero so that He2 molecule does not exist. This molecular orbital treatment can explain why H2 exists but He2 does not. Draw a complete MO diagram for all the bonds in ethene.
Lewis Dot Diagram Helium - schematron.org Lewis Dot Diagrams of Selected Elements The first shell (n=1) can have only 2 electrons, so that shell is filled in helium, the first noble gas. In the periodic. (The exception is helium, He, which only has one energy level or orbital. It is important to remember that Lewis valence dot diagrams are models that show.
Helium Bohr Model - How to draw Bohr diagram for Helium(He ... Here, we will draw the Bohr diagram of the Helium atom with some simple steps. Steps to draw the Bohr Model of Helium atom 1. Find the number of protons, electrons, and neutrons in the Helium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei.
Orbital Diagram For Beryllium A molecular orbital diagram, Beryllium has an electron configuration 1s 2 2s 2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in diberyllium, whereas there are no p orbitals in the valence level of hydrogen or helium. The orbital filling diagram of boron. I skipped past beryllium because I ...
Helium atom - Wikipedia Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force. Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found.
2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
Hydrogen(H) electron configuration and orbital diagram Hydrogen orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction. This is clearly shown in the figure of the orbital diagram of hydrogen. Atomic Orbital Diagram for Hydrogen
Electron configuration - Wikipedia Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
Helium(He) electron configuration and orbital diagram Helium (He) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
HyperPhysics - Georgia State University *helium, liquid *helium-neon laser *Helmholtz free energy *Henry's Law *Hermite polynomials *Hertzsprung-Russell diagram *Heterodyne principle *Higgs boson *Hooke's Law *holography * Hubble constant * Hubble law * hydraulic brakes *hydraulic press *hydrogen bonds *hydrogen radial probability *hydrogen spectrum *hyperbolic functions
Electron configuration for Helium (element 2). Orbital diagram Below is the electronic diagram of the Helium atom Distribution of electrons over energy levels in the He atom 1-st level (K): 2 Valence electrons of Helium The number of valence electrons in a Helium atom - 2. Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
Helium hydride ion - Wikipedia The helium hydride ion or hydridohelium(1+) ion or helonium is a cation (positively charged ion) with chemical formula HeH +.It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang.
Draw the electron configuration diagram for helium? Question Draw the electron configuration diagram for helium? Medium Solution Verified by Toppr Helium has 2 valence electrons, located in period 1, group 18 and in S-block. Therefore its electron configuration is, 1s 2. Was this answer helpful? 0 0 Get the Free Answr app Click a picture with our app and get instant verified solutions Scan Me OR























Comments
Post a Comment