41 bohr diagram for chlorine
What is the Bohr-Rutherford diagram of chlorine? - Answers draw three shells or orbits and then draw 17 electron atoms on the three orbits, two on the first orbit eight on the second or bit and 7... Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Chronic (long-term) exposure to chlorine gas in workers has resulted in respiratory effects, including eye and throat irritation and airflow obstruction.
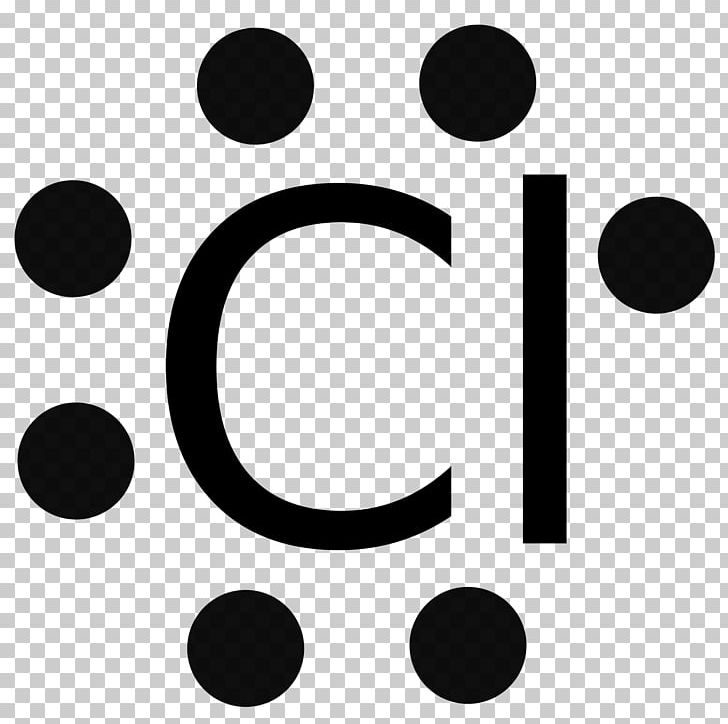
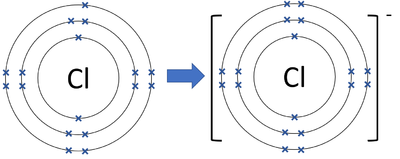
The bohr model diagram represents all the subatomic particles, while the lewis dot diagram only shows the symbol and the valence electrons. ... Q. A student drew a lewis dot structure with 7 valence electrons. Which of the following elements might have the student drawn? answer choices . Cl (Chlorine) Ne (Neon) N (Nitrogen) Li (Lithium) Tags ...

Bohr diagram for chlorine
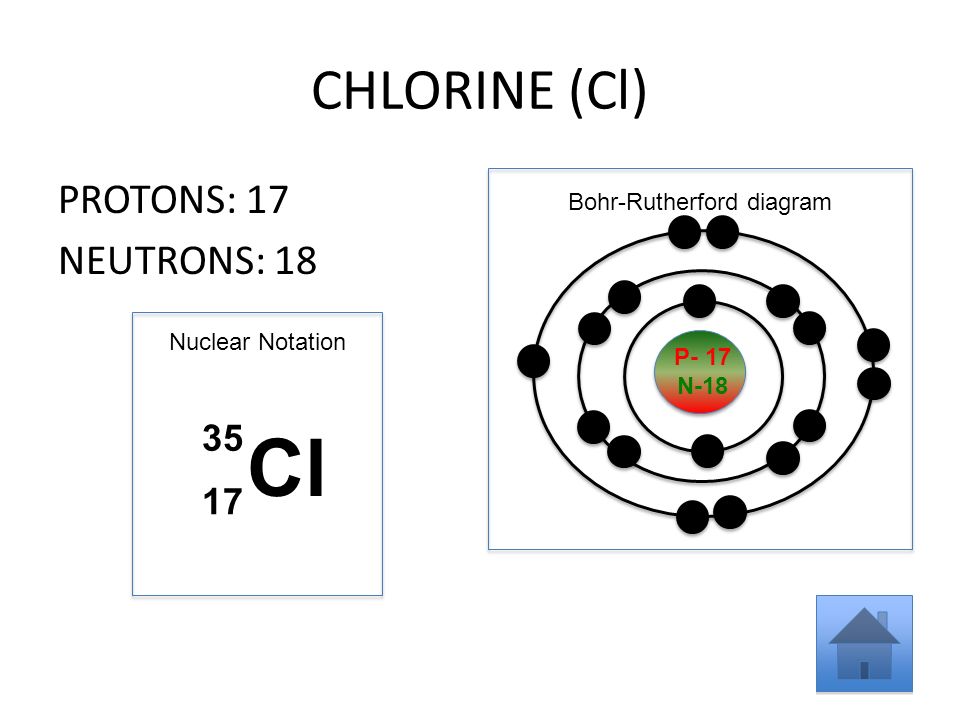
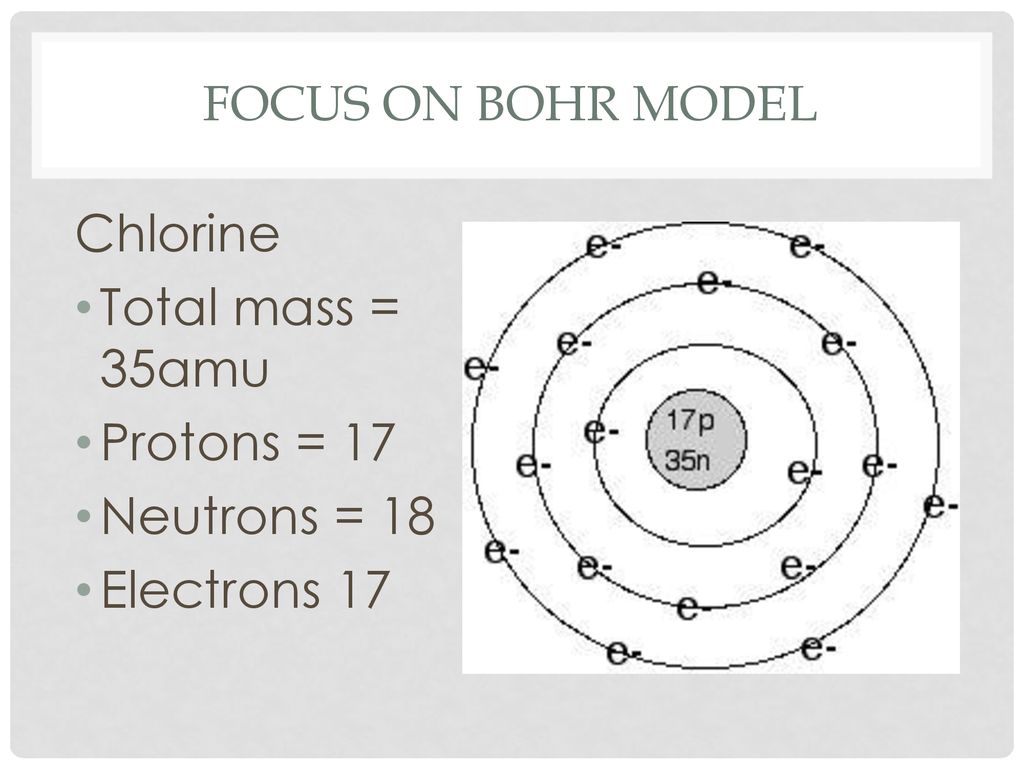
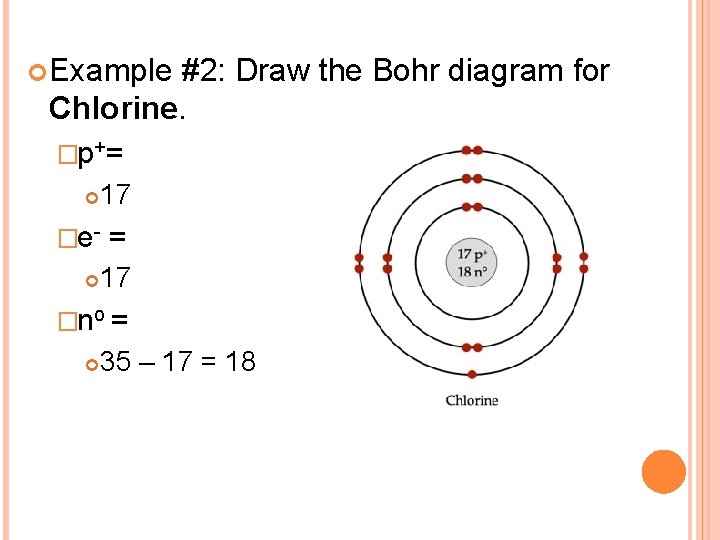
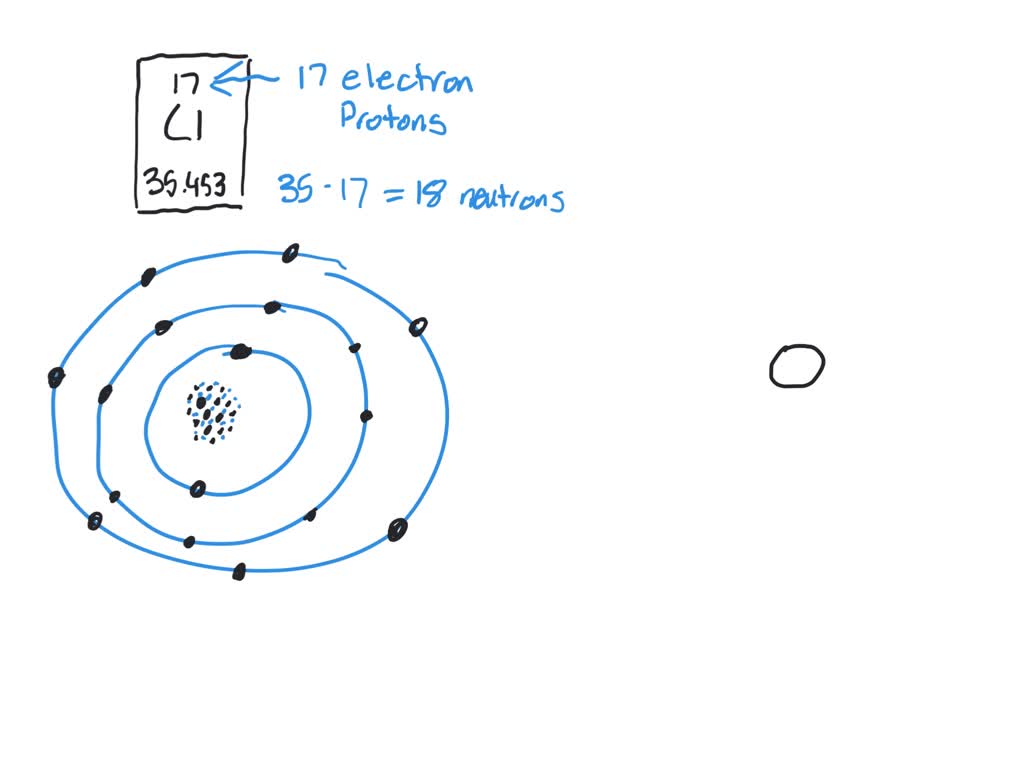
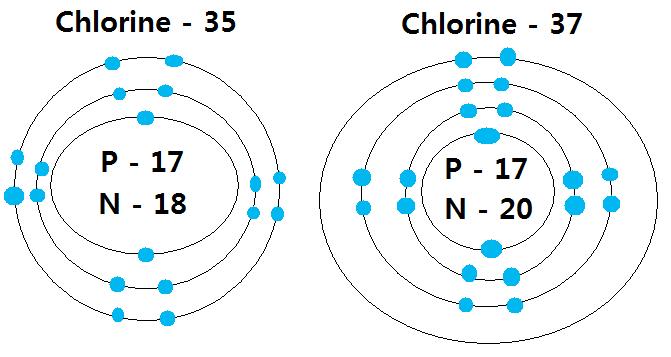
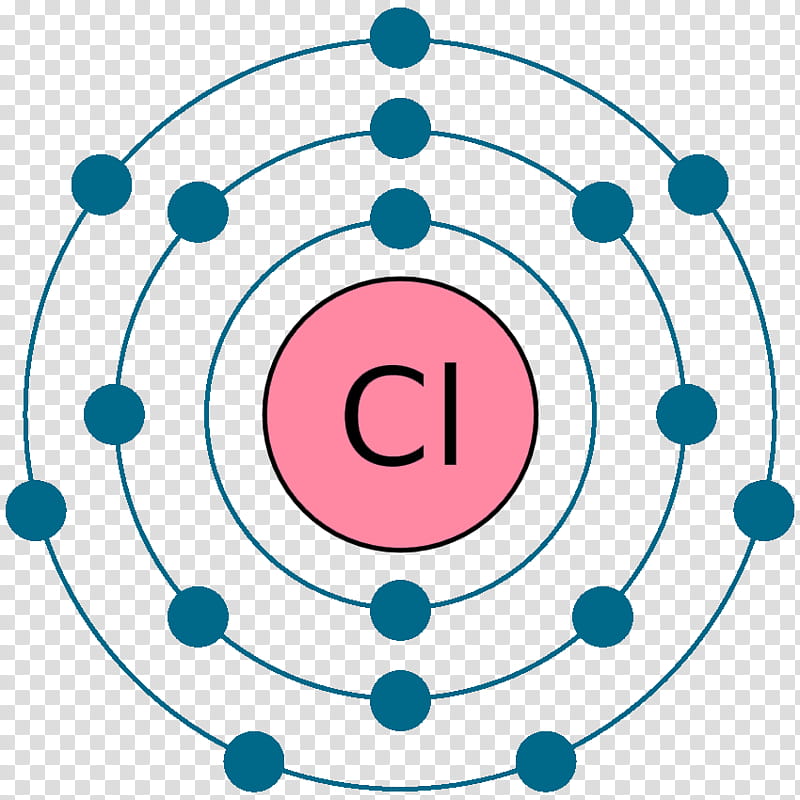
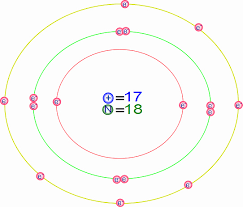
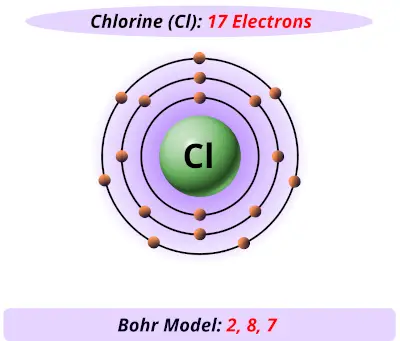
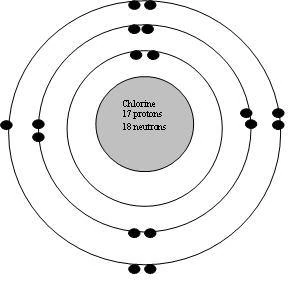
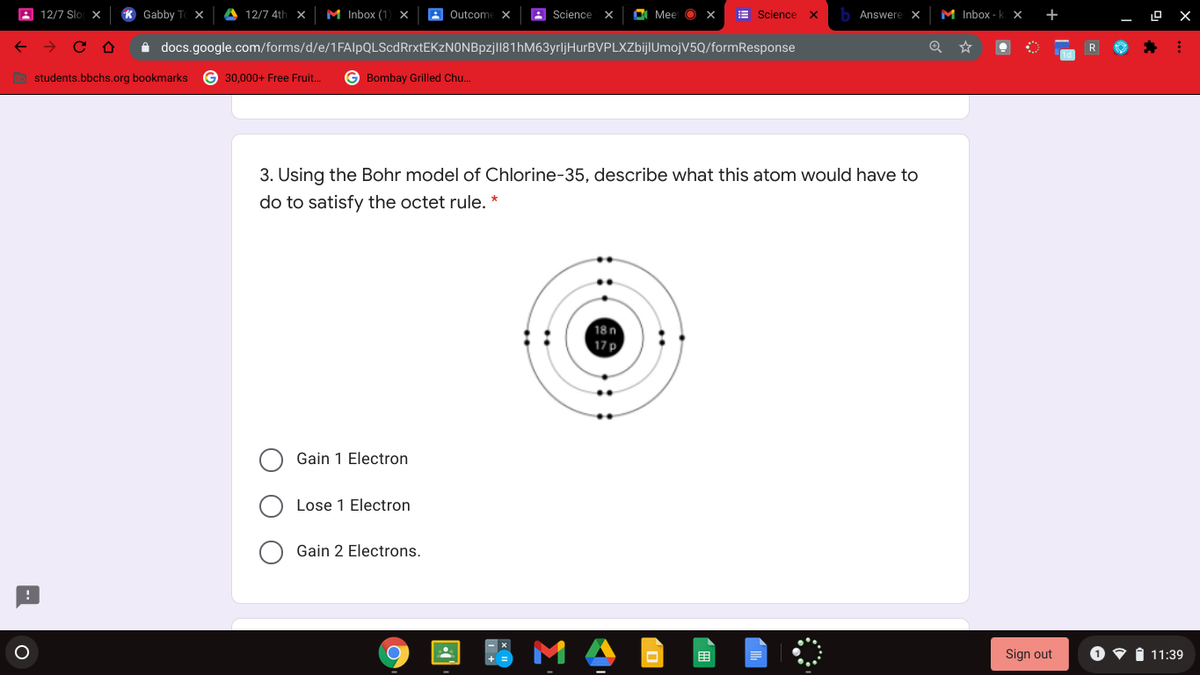
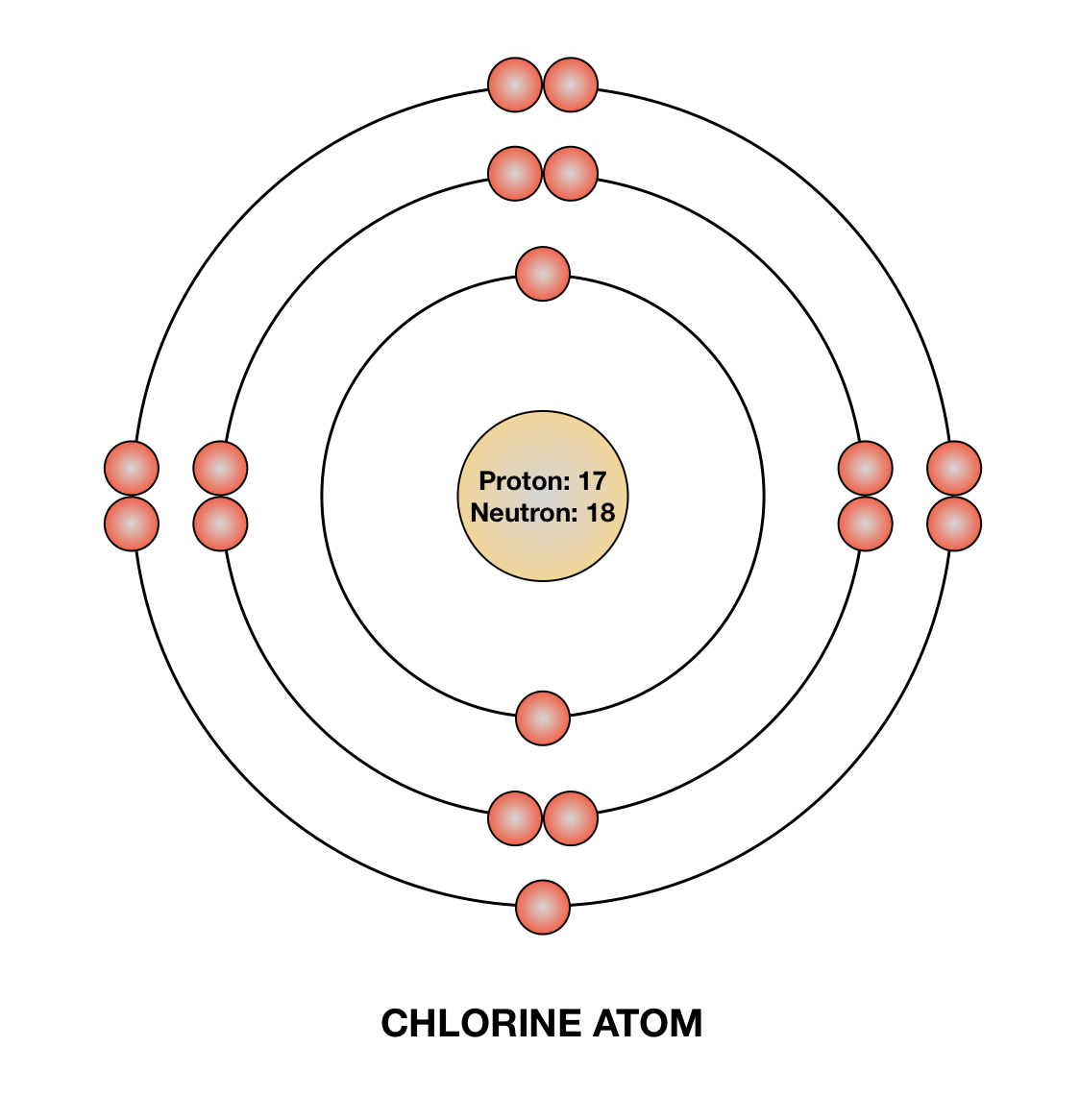
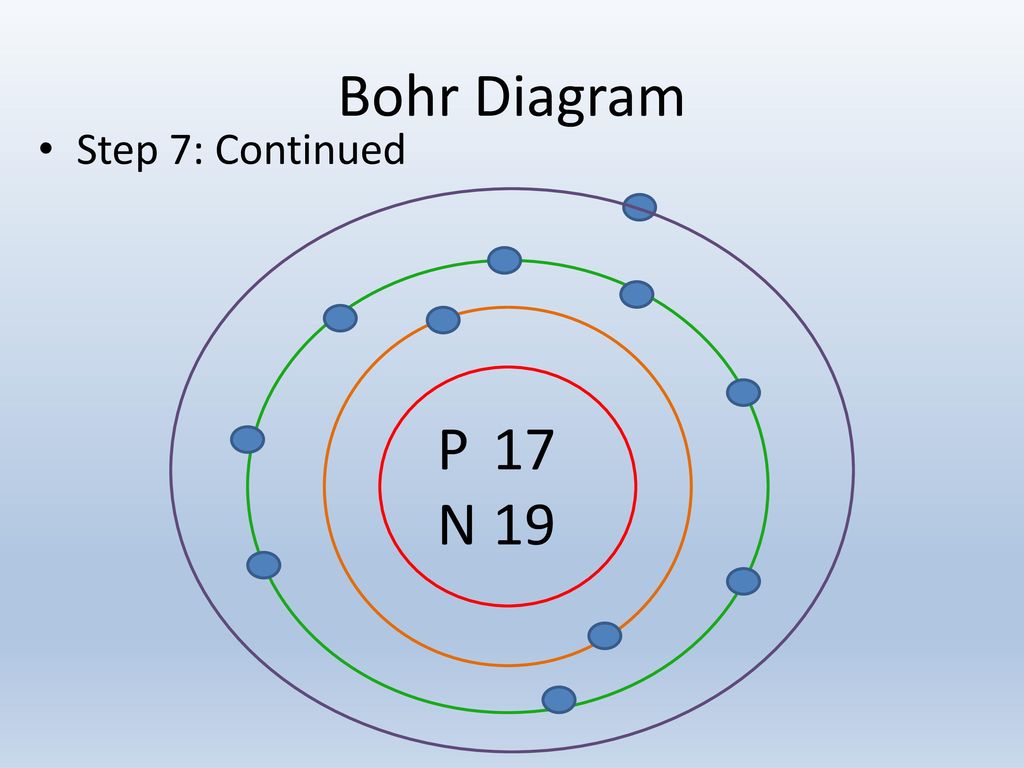
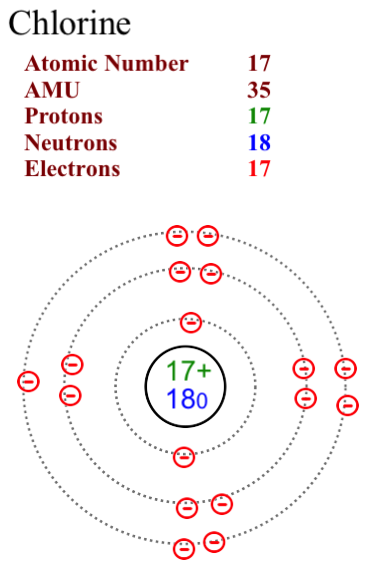
Diagram its electron energy levels using the Bohr model. 8. How many valence electrons does hydrogen have? 9. If carbon has an atomic number of 6 and hydrogen has an atomic number of 1, how many hydrogen atoms can; Question: Part 2 6. Chlorine has an atomic number of 17. Diagram its electron energy levels using the Bohr model. 7. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic structure. The chemical symbol for Chlorine is Cl. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The Bohr Model of Chlorine(Cl) has a nucleus that contains 18 neutrons and 17 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Chlorine contains 7 electrons that also called valence electrons.
Bohr diagram for chlorine. A. The Bohr model did not include positive charges in the atom. B. The Bohr model showed electrons surrounding the positive nucleus. C. The Bohr model consisted of a single, solid mass with no smaller particles. D. The Bohr model showed neutrons in a cloud surrounding the negative nucleus. ____ 39. Which statement is supported by the atomic theory? chlorine atom chlorine ion potassium atom potassium ion Atomic number Number of 10 protons 10 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a neon A step-by-step explanation of how to draw the Lewis dot structure for Cl (Chlorine). I show you where Chlorine is on the periodic table and how to determine... Chlorine Bohr Model — Diagram, Steps To Draw. Chlorine belongs to group 17 of the periodic table. It is a halogen element and occurs as a greenish-yellow gas. It is toxic and corrosive in nature. It naturally occurs in seawater in the form of sodium chloride. Some other chlorine-containing minerals are carnallite, kainite, sylvite, etc.
The Bohr Model of Chlorine(Cl) has a nucleus that contains 18 neutrons and 17 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Chlorine contains 7 electrons that also called valence electrons. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic structure. The chemical symbol for Chlorine is Cl. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Diagram its electron energy levels using the Bohr model. 8. How many valence electrons does hydrogen have? 9. If carbon has an atomic number of 6 and hydrogen has an atomic number of 1, how many hydrogen atoms can; Question: Part 2 6. Chlorine has an atomic number of 17. Diagram its electron energy levels using the Bohr model. 7.
















Comments
Post a Comment