42 orbital diagram boron
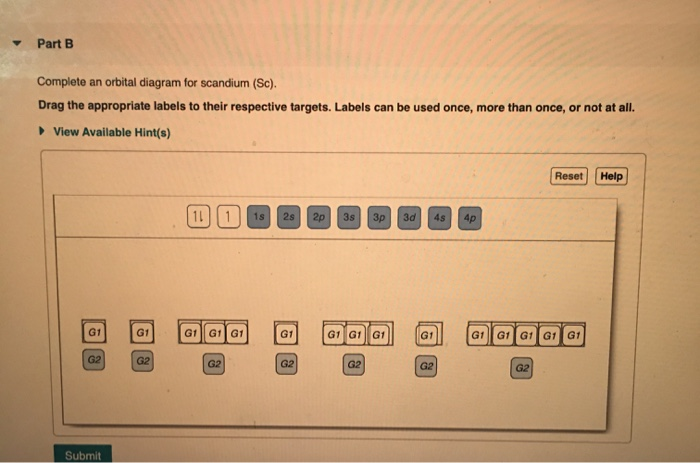
Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of. Question: Draw an orbital diagram for boron. Molecular Orbital Diagram of Boron Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Wa...
To write the orbital diagram for the Boron atom (B) first we need to write the electron configuration for just B. To do that we need to find the number of e...

Orbital diagram boron
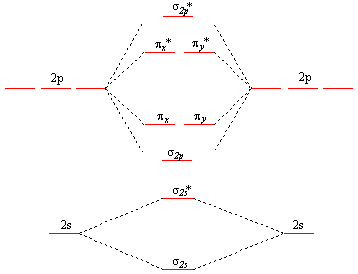
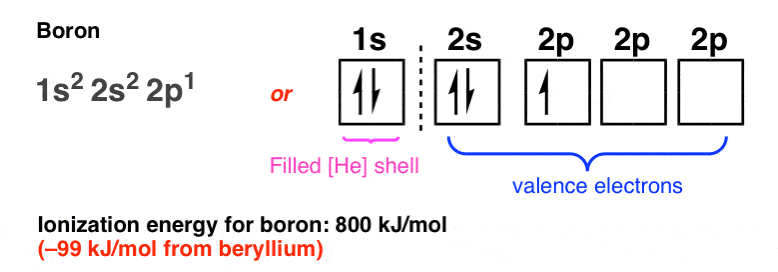
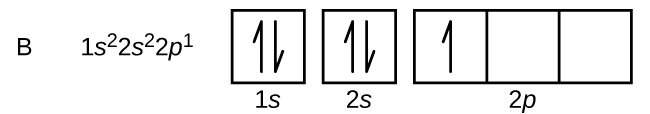
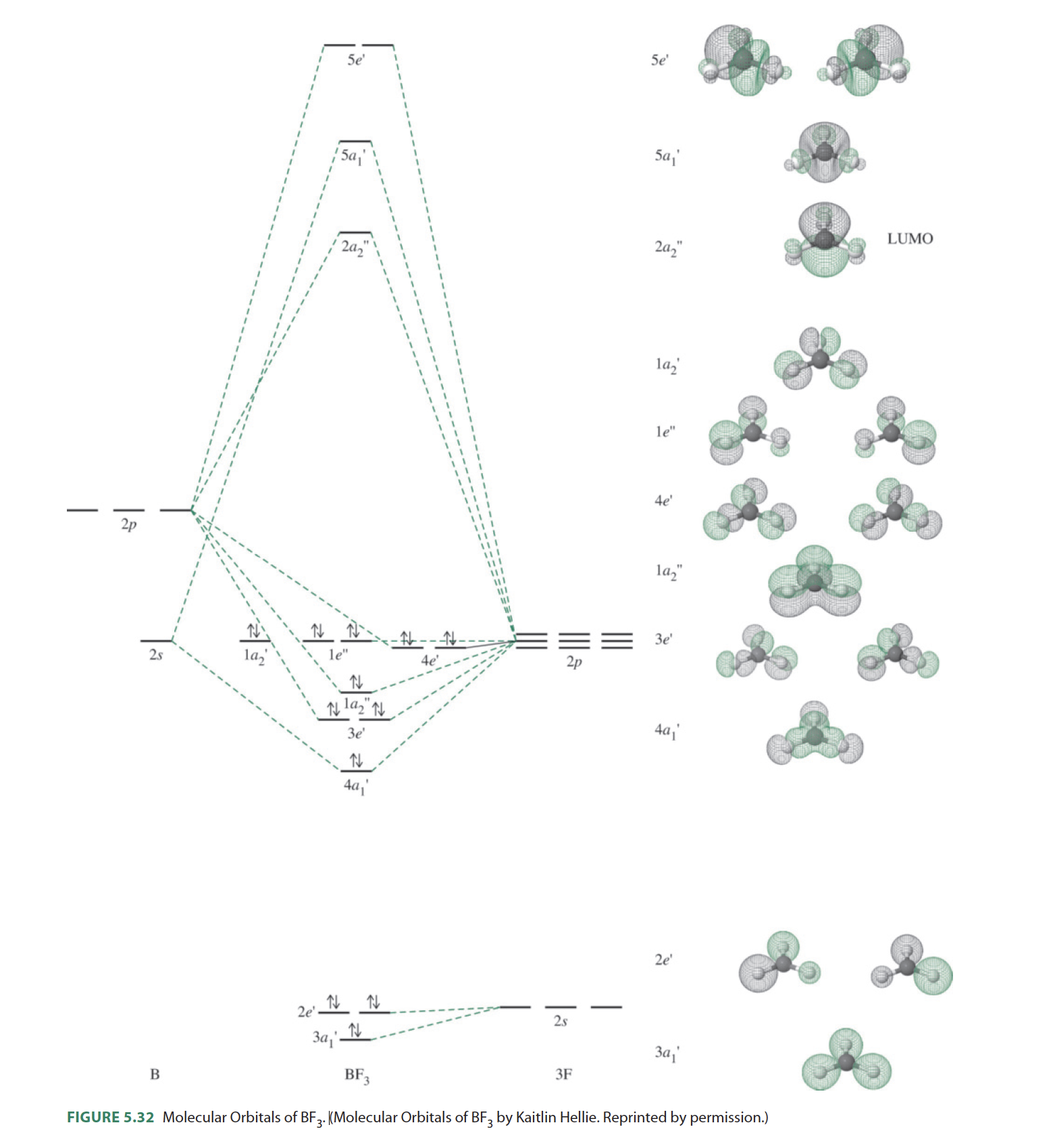
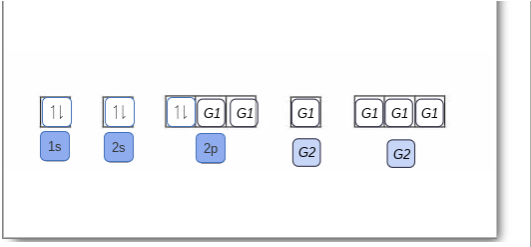
Electron configuration of boron(B) atom through orbital diagram. Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, f. Complete an orbital diagram for boron. Boron is the fifth element with a total of 5 electrons. Use this tool to draw the orbital diagram. Therefore the b electron configuration will be 1s22s22p1. Lower energy subshells fill before higher energy subshells. Use the buttons at the top of the tool to add orbitals. Mar 18, 2019 · A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Electron Configuration for Boron (B)Electron Configuration for Boron (B)
Orbital diagram boron. In this video, we will explore the rules to follow when drawing orbital diagrams. We also determine the orbital diagram of boron. This problem has been solved! See the answer. See the answer See the answer done loading. Complete an orbital diagram for boron. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. 1s2, 2s2, 2p1 Boron 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d1. Scandium. Answer to Draw an orbital diagram for boron. Use this tool to draw the orbital diagram. Draw an orbital diagram for ... Q: Orbital filling diagram of boron. Write your answer... Submit. Related questions. Which is the correct orbital diagram for boron? B. What is the orbital filling diagram for C? 1s2, 2s2 2p2.
Nov 01, 2021 · Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine ... Complete an orbital diagram for boron. Draw orbital diagrams, and use them to derive electron configurations. To understand how to draw orbital diagrams, and how they are used to write electron configurations. The electron configuration of an element is the arrangment of its electrons in their atomic orbitals. Mar 18, 2019 · A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Electron Configuration for Boron (B)Electron Configuration for Boron (B) Complete an orbital diagram for boron. Boron is the fifth element with a total of 5 electrons. Use this tool to draw the orbital diagram. Therefore the b electron configuration will be 1s22s22p1. Lower energy subshells fill before higher energy subshells. Use the buttons at the top of the tool to add orbitals.
Electron configuration of boron(B) atom through orbital diagram. Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, f.
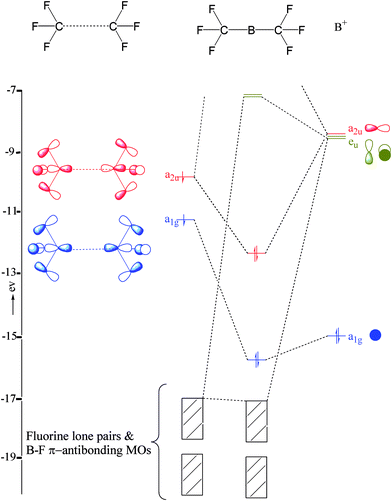
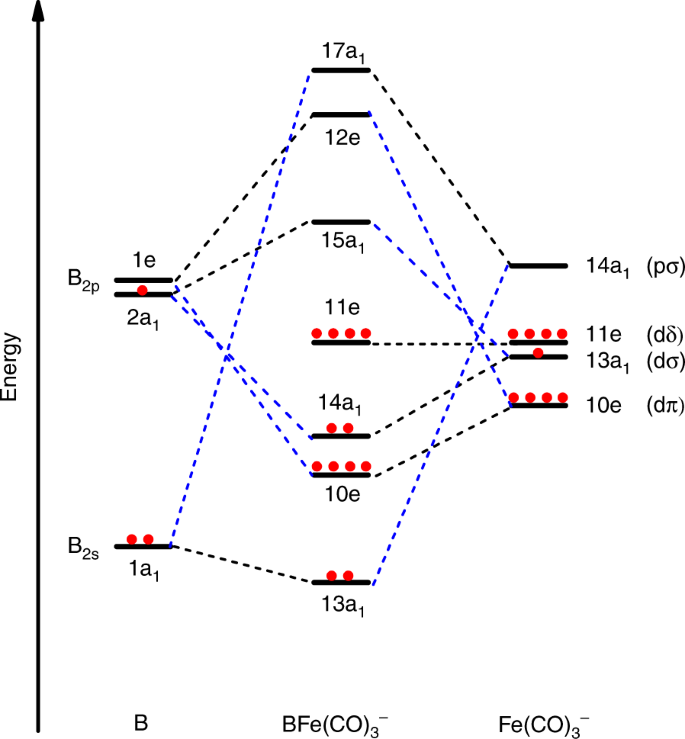
Structure And Bonding In Boron Carbide The Invincibility Of Imperfections New Journal Of Chemistry Rsc Publishing Doi 10 1039 B618493f




















Comments
Post a Comment